Abstract
Background : Although total body irradiation (TBI)-based ablative conditioning regimens prior to allogeneic stem cell transplantation (alloHCT) have been demonstrated to be efficacious in multiple hematologic malignancies, the relapse rate and survival outcomes for relapsed/refractory (R/R) acute leukemia remain poor due in large part to limited disease control and high non-relapse mortality, which over time has led to the exclusion of these patients from such treatment. In one report, standard myeloablative alloHCT regimens, frequently in combination with TBI, yielded 1-year overall survival (OS) of only 29% and 28% for active acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), respectively (Duval et al., J Clin Oncol, 2010). In response to these limitations, our group in a phase I trial reported that replacing TBI with targeted conformal total marrow and lymphoid irradiation (TMLI) at 2000 cGy as a conditioning regimen with cyclophosphamide (Cy) and etoposide (VP16) is safe in patients with R/R AML and ALL. Here, we report the results to date of a phase II study in which we combined the recommended dose of TMLI (2000 cGy) in combination with Cy and VP16. The objective is to evaluate the anti-leukemia activity and safety/tolerability of the TMLI/Cy/VP16 alloHCT conditioning regimen in patients with R/R acute leukemia.
Patients and Methods : TMLI was administered on days -9 to -5, VP16 60 mg/kg (adjusted body weight) on day -4, and Cy 100 mg/kg (ideal body weight) on day -2. The radiation dose for all patients (n=35) was 2000 cGy, delivered in 200 cGy fractions twice daily. The radiation dose delivered to the liver and brain was kept at 1200 cGy. Bone marrow (n=2) or peripheral blood stem cells (n=33) were given on day 0. The primary endpoint is progression free survival (PFS); secondary endpoints include overall survival (OS), non-relapse mortality (NRM), and toxicity. Tacrolimus and sirolimus were administered for graft versus host disease (GVHD) prophylaxis. Toxicities were defined according to the Bearman and CTCAE 4.03 scales, the latter for hematologic toxicity. Stopping rules for unacceptable adverse events (AEs) were incorporated; the rate of unacceptable toxicity should not be ≥33% in the first 30 days post stem cell infusion.
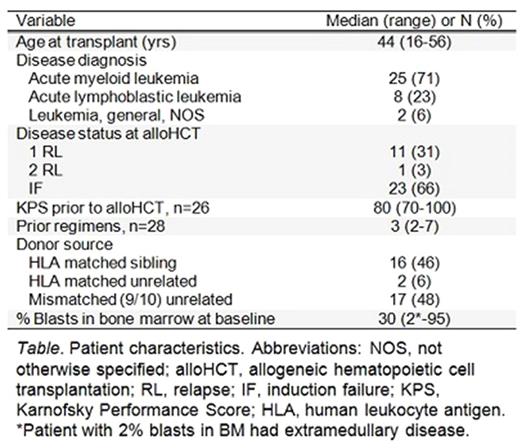
Results : A total of 35 patients were enrolled and treated (see Table) between 5/21/2014 and 12/14/2016, with a median follow up of 11.8 months (range 1.8-35.9) for all patients and 12.0 months (range 2.9-35.9) for surviving patients (n=17). All patients engrafted. The complete remission rate at day +30 was 100%. One-year estimates of OS and PFS were 62.4% (95% CI: 43.1-76.8) and 44.7% (95% CI: 27.1-60.9), respectively. Disease relapse/progression at 1 year was 52.4% (95% CI: 37.6-73.2). The estimates of NRM at 100 days and 1 year were both 2.9% (95% CI: 0.4-19.7). Relapsed disease after transplant occurred in 21 patients (60%). Two patients died in remission; the causes were West Nile encephalitis and infection, respectively.
Acute GVHD (aGVHD) developed in 20 (57%) of patients; of those, only 2 patients (6%) developed grades 3-4. Bearman toxicity data are available for 30 patients. Among these patients, grade ≥2 toxicities were bladder (Gr 2 n=1), central nervous system (Gr 2 n=1), gastrointestinal (Gr 2 n=4), hepatic (Gr 2 n=1, Gr 3 n=1), pulmonary (Gr 2 n=1), and renal (Gr 2 =1, Gr 3 n=1). Additionally, 11 and 1 patients experienced grade 2 and 3 stomatitis, respectively. No grade 4 toxicities or toxicity-related deaths were observed. The stopping rules for unacceptable AEs were not met.
Conclusions : 1) TMLI allows a dose of 2000 cGy to be safely delivered to targeted sites, with a 100 day and 1 year NRM of 2.9% in this high-risk patient population; 2) One-year OS and PFS are very encouraging in patients with R/R acute leukemia; 3) Toxicities and acute GVHD were manageable and in line with previous experience with this regimen.
Stein: Amgen: Consultancy, Speakers Bureau; Stemline: Consultancy. Khaled: City of Hope: Research Funding; Daiichi Sankyo, Inc: Other: Travel Support. Salhotra: Kadmon: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal